We aim to produce high yields of novel HBV/HCV antigens with superior immunogenic properties in plants and mammalian cells, based on innovative molecular design and establish in premiere an advanced biotechnological platform for production of best vaccine candidates antigens in algae.

Hepatitis B (HBV) and C viruses (HCV) infect the human liver, triggering persistent inflammation and eventually cirrhosis and hepatocellular carcinoma (HCC), the second leading cause of cancer-related mortality worldwide. Currently, more than 500 million people are chronically infected with HBV or HCV and at high risk of developing end stage liver disease and HCC. Collectively, HBV and HCV infections are responsible for about 1.3 million deaths annually. HBV infection is not curable with the treatment available today due to the nuclear replication form not being eliminated from infected hepatocytes. There is no doubt that vaccination remains the most effective strategy to limit HBV spreading and control the disease. Administration of the standard recombinant vaccine containing the small (S) HBV envelope protein produced in yeast (the HBsAg) had a major impact on HBV-related liver disease and death. Unfortunately, about 10% of vaccinated people do not develop protective antibody titers and remain vulnerable to infection. Moreover, the duration of the immunity conferred by the 3-dose intramuscular administration of the HBsAg is unclear, several studies indicating that administration of booster doses might be needed at 15 years post primary vaccination. A strategy to overcome these issues has considered the development of improved adjuvants. Other approaches have investigated the possibility to incorporate the more immunogenic middle (M) and large (L) HBV envelope proteins in the standard vaccine. However, the high costs associated with expression of these proteins in mammalian cells prevented the widespread use of the novel generation of HBV vaccines.
In the case of HCV, innovative direct acting antiviral cocktails which can achieve sustained virological response in more than 90% of treated patients have considerably advanced current therapy. Despite this success, the high treatment costs, the emergence of resistant mutants and the susceptibility to reinfection, underline the urgency for the development of a protective HCV vaccine that is still missing. Early clinical trials are ongoing for prophylactic B and T-cell based vaccines. A vaccine formulation based on HCV envelope proteins E1E2 heterodimer elicited cross-neutralizing antibodies in a minority of immunized patients despite its efficacy in animals. Although there is proof of concept in humans that HCV vaccination is possible, the design of the ideal antigen able to trigger protective immune response across HCV genotypes remains a major scientific challenge in the field.
The ultimate goal of prophylactic treatment in HBV and HCV infection is virus eradication, which can be achieved by conducting universal vaccination programs. However, these programs are financially very challenging and most low-income countries, which are confronted with the highest rates of infection, cannot afford them. Production of cost-effective vaccines would alleviate the economic burden on public health systems and increase availability to vulnerable societies.
In this context, our project proposal aims to: a) build upon our experience and knowledge accumulated in our previous collaboration on plant expression of HBV/HCV proteins (GreenVac project 2014-2017 funded by the EEA Norway-Romania Program) and produce high yields of novel HBV/HCV antigens with superior immunogenic properties based on innovative molecular design; b) take this experience a step forward and establish in premiere an advanced biotechnological platform for production of HBV/HCV antigens in algae; c) take advantage of the multiple recombinant protein expression systems developed in the consortium and perform systematic, comparative characterization of the biochemical and functional properties of the new antigens in relation to their immunogenicity; d) develop institutional networking around innovative plant and microalgae biotechnologies and train human resources in protein science in applied research in Romania and Norway.
Virusurile Hepatice B (VHB) si C (VHC) infecteaza ficatul uman, provocand inflamatie persistenta, ciroza si carcinom hepatocelular (CHC), a doua cauza de mortalitate datorata cancerului din lume. Mai mult de 500 milioane de oameni sunt astazi infectati cronic cu VHB sau VHC, prezentand un risc crescut de a dezvolta boli hepatice terminale si CHC. Impreuna, infectiile VHB si VHC sunt responsabile de moartea a aproape 1,3 milioane de pacienti in fiecare an. Tratamentele disponibile in mod curent nu vindeca infectia cu VHB, din cauza formei nucleare de replicare, care nu este eliminata din hepatocitele infectate. Astfel, vaccinarea ramane cea mai eficienta strategie pentru controlul infectiei VHB si limitarea aparitiei de cazuri noi. Administrarea vaccinului standard recombinat, care contine proteina de invelis a VHB „small” (S) produsa in drojdii (HBsAg) a avut un impact major asupra patologiei hepatice si mortalitatii datorate VHB.Totusi, aproximativ 10% din persoanele vaccinate nu dezvolta anticorpi cu un titru protector, ramanand vulnerabili infectiei. Mai mult durata raspunsului imun obtinut prin administrarea intramusculara a HbsAg in trei doze nu este foarte clara, existand studii care sugereaza necesitatea administrarii unei doze de rapel dupa 15 ani de la vaccinarea initiala. O strategie adoptata pentru depasirea acestor probleme a luat in considerare dezvoltarea de adjuvanti mai eficienti. Alte strategii au investigat posibilitatea introducerii celorlalte doua proteine de invelis a VHB, „middle” (M) si „ large” (L), care sunt mai imunogene, in vaccinul standard. Totusi, costurile mari asociate producerii acestor proteine in culturi de celule mamaliene au impiedicat folosirea la scala larga a acestei noi generatii de vacinuri anti-VHB.
In cazul VHC, terapiile inovatoare care presupun administrarea de amestecuri de inhibitori cu actiune antivirala directa sunt foarte eficiente, determinand eliminarea infectiei in mai mult de 90% din cazuri. In ciuda acestui succes, costurile ridicate ale tratamentului, aparitia unor mutatii rezistente si posibilitatea reinfectiei sunt argumente pentru dezvoltarea unui vaccin profilactic anti-VHC, care nu este inca disponibil. Exista insa numeroase studii clinice in desfasurare care analizeaza diferite formule vaccinale bazate pe stimularea celulelor B si T. Una dintre acestea, bazata pe includerea heterodimerului E1E2 al VHC a indus anticorpi cu capacitate de neutralizare incrucisata intr-o minoritate de pacienti vaccinati, in ciuda unei eficiente mai mari in animale. Astfel, desi studiile clinice confirma validitatea conceptului vaccinarii impotriva VHC, dezvoltarea antigenului ideal, capabil sa induca un raspuns imun incrucisat fata de diferitele genotipuri VHC, ramane o provocare stiintifica majora in domeniu.
Principala tinta in tratamentul profilactic al infectiilor cu VHB si VHC ramane eradicarea virusurilor, care ar putea fi posibila prin aplicarea unor programe de vaccinare universala. Aceste programe reprezinta o provocare din punct de vedere financiar, in special in tarile cu o economie slab –dezvoltata, care se confrunta cu cele mai mari rate de infectie si nu-si pot permite vaccinarea in masa. Producerea unor vaccinuri mai ieftine ar reduce povara economica din sistemele de sanatate publica si ar creste accesul la tratament in societatile cele mai vulnerabile.
In acest context, obiectivele proiectului nostru sunt: a) dezvoltarea experientei si cunoasterii asupra producerii proteinelor VHB/VHC in plante, acumulate in colaborarea anterioara (Proiectul GreenVac 2014-2017 finantat prin programul SEE Norvegia-Romania) si producerea unor antigene VHB/VHC cu randament si proprietati imunologice superioare, printr-un design molecular inovativ; b) extinderea acestei experiente prin dezvoltarea unei platforme biotehnologice avansate de producere, in premiera, a antigenelor VHB/VHC in alge; c) caracterizarea sistematica, comparativa a proprietatilor biochimice si functionale ale noilor antigene produse in diferitele sisteme de expresie de proteine, disponibile in consortiu, in relatie cu caracterul lor imunogenic; d) dezvoltarea unei retele institutionale de biotehnologii inovative in plante si microalge si calificarea de noi resurse umane in stiinta proteinelor si cercetare aplicativa, in Romania si Norvegia.
Publications in the frame of the project
2020
1.N-Glycosylation and N-Glycan Processing in HBV Biology and Pathogenesis. Mihaela-Olivia Dobrica, Catalin Lazar and Norica Branza-Nichita. Cells 2020, 9, 1404; doi:10.3390/cells9061404, IF 5,6
2. A polycarboxylic chelating ligand for efficient resin purification of His-tagged proteins expressed in mammalian systems. Codruta C. Popescu, Marius C. Stoian, Lia-Maria Cucos, Anca G. Coman, Antonio Radoi, Anca Paun, Niculina D. Hadade, Arnaud Gautier, Costin-Ioan Popescu and Mihaela Matache. RSC Adv., 2020, 10, 23931, IF 3, 09
3. Production of Chimeric Hepatitis B Virus Surface Antigens in Mammalian Cells. Mihaela-Olivia Dobrica, Catalin Lazar and Norica Branza-Nichita. Vaccine Delivery Technology: Methods and Protocols, Methods in Molecular Biology, Blaine Pfeifer and Andrew Hill (eds.), vol. 2183, https://doi.org/10.1007/978-1-0716-0795-4_7, © Springer Science+Business Media, LLC, part of Springer Nature. Book chapter.
Publicatii in cadrul proiectului:
2020
1.N-Glycosylation and N-Glycan Processing in HBV Biology and Pathogenesis. Mihaela-Olivia Dobrica, Catalin Lazar and Norica Branza-Nichita. Cells 2020, 9, 1404; doi:10.3390/cells9061404, IF 5,6
2. A polycarboxylic chelating ligand for efficient resin purification of His-tagged proteins expressed in mammalian systems. Codruta C. Popescu, Marius C. Stoian, Lia-Maria Cucos, Anca G. Coman, Antonio Radoi, Anca Paun, Niculina D. Hadade, Arnaud Gautier, Costin-Ioan Popescu and Mihaela Matache. RSC Adv., 2020, 10, 23931, IF 3, 09
3. Production of Chimeric Hepatitis B Virus Surface Antigens in Mammalian Cells. Mihaela-Olivia Dobrica, Catalin Lazar and Norica Branza-Nichita. Vaccine Delivery Technology: Methods and Protocols, Methods in Molecular Biology, Blaine Pfeifer and Andrew Hill (eds.), vol. 2183, https://doi.org/10.1007/978-1-0716-0795-4_7, © Springer Science+Business Media, LLC, part of Springer Nature. Book chapter.
In perioada 20-21 August 2020, a avut loc prima intalnire anuala SmartVac, in format online.
1st SmartVac videoconference



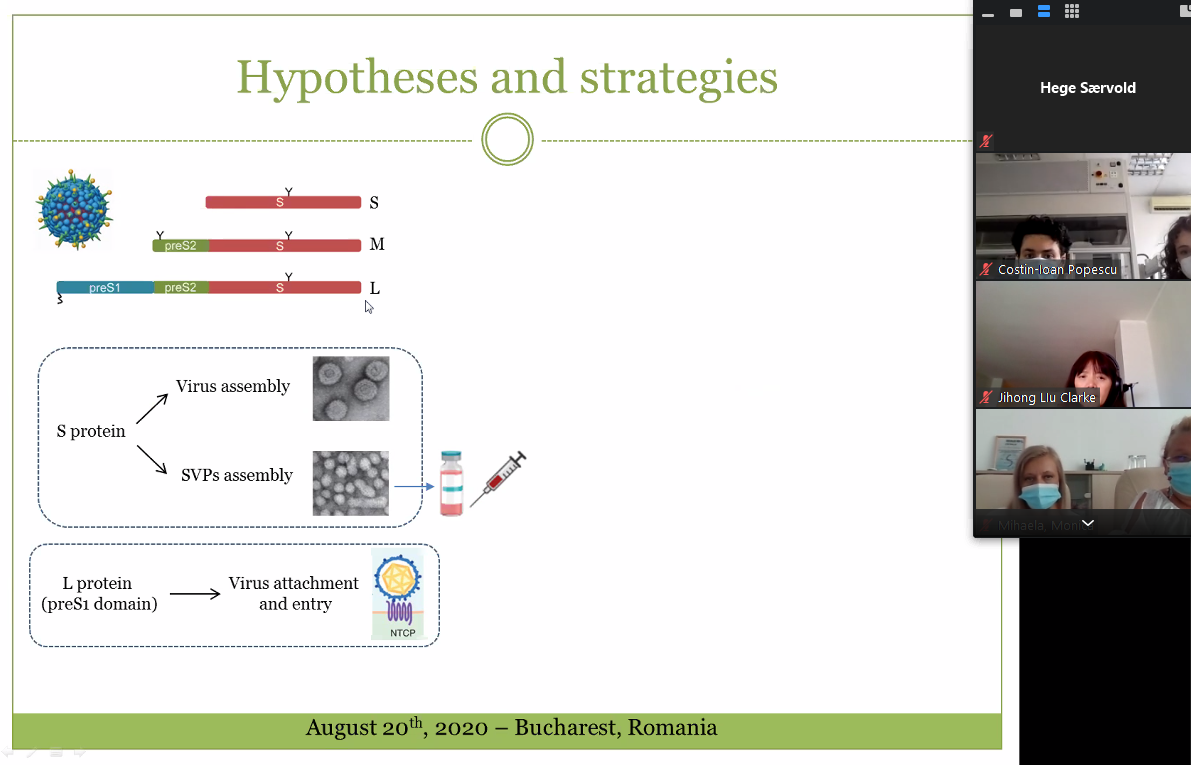
In perioada 28-30 Octombrie membrii proiectului SmartVac din cadrul Institutului de Biochimie, s-au intalnit in As, Norvegia, cu partnerii din NIBIO, pentru conferinta de deschidere a proiectului.
Kick-off meeting Agenda


Project presentation held by Dr. Nichita at NIBIO in Norway in October 2019

Romanian, Norwegian, and German project representatives meeting

Representatives group photo

The EEA Grants represent the contribution of Iceland, Liechtenstein and Norway towards a green, competitive and inclusive Europe.
There are two overall objectives: reduction of economic and social disparities in Europe, and to strengthen bilateral relations between the donor countries and 15 EU countries in Central and Southern Europe and the Baltics.
The three donor countries cooperate closely with the EU through the Agreement on the European Economic Area (EEA). The donors have provided €3.3 billion through consecutive grant schemes between 1994 and 2014. For the period 2014-2021, the EEA Grants amount to €1.55 billion. The priorities for this period are:
#1 Innovation, Research, Education and Competitiveness
#2 Social Inclusion, Youth Employment and Poverty Reduction
#3 Environment, Energy, Climate Change and Low Carbon Economy
#4 Culture, Civil Society, Good Governance and Fundamental Rights
#5 Justice and Home Affairs
The EEA Grants are jointly financed by Iceland, Liechtenstein and Norway, whose contributions are
based on their GDP. Eligibility for the Grants mirror the criteria set for the EU Cohesion Fund aimed at member countries where the Gross National Income (GNI) per inhabitant is less than 90% of the EU average.
News release NIBIO site: https://www.nibio.no/en/news/renewed-funding-for-vaccine-research